HIPAA vs. GDPR vs. CAN-SPAM: Navigating B2B Data Licensing in 2026
If you are buying a B2B contact list today, you aren’t just buying names and emails. You are buying a legal responsibility. One wrong move with a healthcare provider (HCP) list or an EU-based database can lead to fines that make your quarterly budget look like pocket change.
The rules of the game shifted recently. HIPAA just underwent a major update regarding substance use disorder (SUD) records, and GDPR regulators are tightening the definition of “personal data” in a B2B context. Here is exactly how to differentiate these three frameworks before you sign your next licensing contract.
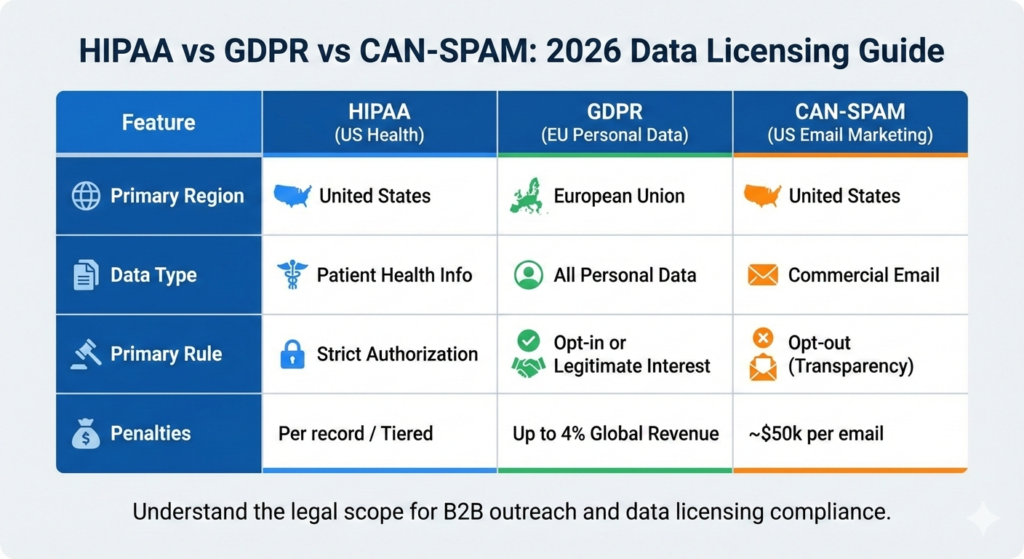
What is the difference between HIPAA, GDPR, and CAN-SPAM?
HIPAA (Health Insurance Portability and Accountability Act) protects patient health data in the US, ensuring privacy in healthcare settings. GDPR (General Data Protection Regulation) is a broad EU law protecting all personal data of individuals within the EU, regardless of industry. CAN-SPAM is a US federal law that sets the ground rules for commercial email marketing, focusing on transparency and the right to opt out.
The Feb 2026 HIPAA Update: SUD Records
As of February 16, 2026, the HIPAA Privacy Rule (specifically 42 CFR Part 2) is fully aligned with standard healthcare operations.
If your licensed data touches on substance use disorder history, you no longer need to physically segregate these records in your CRM. However, you must now implement granular, role-based access. For B2B licensors, this means your provider’s Business Associate Agreement (BAA) must specifically address these 2026 updates to ensure your “minimum necessary” data usage is legally defensible.
2. GDPR: Why Work Emails Are “Personal”
A frequent error in B2B outreach is the belief that a business email like jane.doe@enterprise.com is exempt from privacy laws. Under GDPR, any identifier tied to a specific person is personal data.
If you license data for EU prospects, you must have a “lawful basis.” Most B2B firms use “Legitimate Interest,” but this requires a documented Legitimate Interest Assessment (LIA). You must also provide an immediate, “one-click” way for them to be forgotten.
3. CAN-SPAM: The $53,088 Risk
While GDPR focuses on your right to hold data, CAN-SPAM focuses on your behavior. As of early 2026, the fine for a single non-compliant email has risen to $53,088.
If you license a US-based list, your outreach must include:
- A valid physical postal address.
- Clear identification that the message is an advertisement.
- An opt-out mechanism that you honor within 10 business days.
4. Is B2B Licensed Data HIPAA Compliant?
HIPAA usually does not apply to standard B2B contact info (names, titles) unless it is linked to Protected Health Information (PHI). However, if your licensing vendor sources data from patient records or clinical outcomes, you must ensure they follow the “minimum necessary” rule and have a valid BAA in place.
5. Can I Cold Email Under GDPR in 2026?
Yes, if you rely on “legitimate interest”. You must ensure the outreach is highly relevant to the recipient’s professional role and provide a clear privacy notice in the first email. You are also required to delete their data immediately if they exercise their “right to be forgotten”.
6. Reputation is Your Compliance Shield
Regulators and spam filters often target senders with high complaint rates. When you license data, accuracy is your best defense. Sending emails to “dead” healthcare addresses triggers security gateways that flag your domain.
Because healthcare servers often use complex configurations, standard tools frequently fail. For high-stakes 2026 campaigns, using a catch-all verifier is the only way to protect your sender reputation and prove you are acting with “due diligence” in your outreach.
7. Data Accuracy: The Secret to Staying Under the Radar
Compliance isn’t just about legal frameworks; it’s about data hygiene. Sending emails to “dead” addresses or outdated HCP roles triggers spam filters and manual complaints. Industry standards now demand 95%+ accuracy for quality lists.
When licensing data, we have found that verifying catch-all emails is the most ignored step. Because many enterprise and healthcare servers use “catch-all” configurations, traditional verifiers often fail.
8. Real-World Scenario: The Pharma Launch
Imagine a medical device company licensing a list of 50,000 orthopedic surgeons.
- The HIPAA Angle: The list itself is just professional data. But if the company tracks which surgeons click a link about a specific patient-case study, they might be handling PHI.
- The GDPR Angle: If 5,000 of those surgeons are in Germany, the company must have an LIA on file and a clear privacy notice.
- The CAN-SPAM Angle: Every email must have the company’s physical office address and an unsubscribe link that works within 10 days.
9. Benchmarks for Success
In the healthcare sector, quality data licensing pays off. Average B2B healthcare campaigns see a 36.23% open rate when the data is fresh and the targeting is specific. Geographic targeting alone can boost response rates by 40% because it aligns with local state-level health regulations.
10. Choosing a Compliant Partner
Don’t just take a vendor’s word for it. Ask for:
- SOC-2 or ISO 27001 Certification: This proves they have technical safeguards.
- Source Transparency: They should tell you exactly how the data was gathered (e.g., opt-in forms, public registries).
- Regular Audits: Ensure they clean their database at least quarterly.
11. Does CAN-SPAM Require “Double Opt-In”?
PAA Question: Do I need a double opt-in for US email marketing? Answer: No, the CAN-SPAM Act does not require a double opt-in process for commercial emails. You can send a cold email to a business contact without prior consent, provided you identify the message as an advertisement, include your physical address, and provide a clear opt-out mechanism.
12. Conclusion: Priorities for 2026
Data licensing is a powerful tool, but it requires a disciplined approach to governance.
- Update your BAAs to reflect the Feb 2026 HIPAA SUD changes.
- Audit your EU lists for “legitimate interest” documentation.
- Clean your data to maintain that 95% accuracy benchmark.
Ready to clean your list and stay compliant? Start by verifying those tricky healthcare emails to ensure your deliverability stays high.
Author and Experience
Written by: SparkDBi Customer Success Team
Experience context: Based on aggregating 270 Million+ global business data points, healthcare HCP data, and 2,000+ outbound campaigns, millions of email verifications, and enterprise GTM data activations across multiple industries including SaaS and healthcare.
